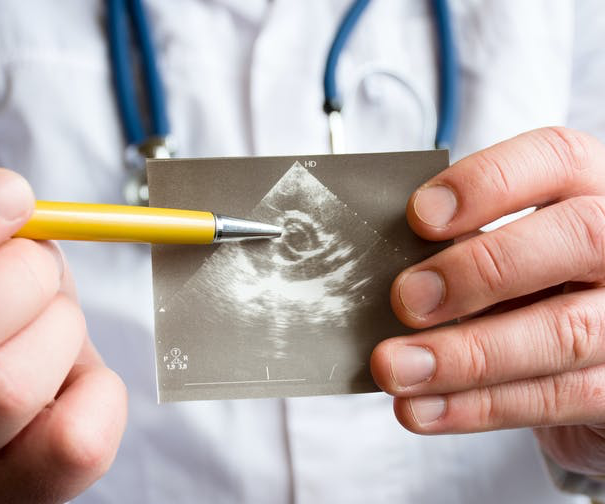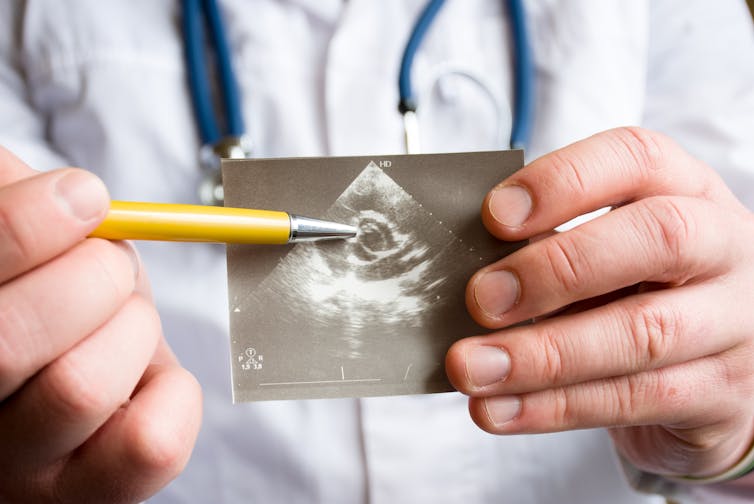
An ultrasound of a heart indicating possible pathology of heart aortic valve. (Shutterstock) Marta Cerruti, McGill University and Ophélie Gourgas, McGill University
Heart disease is the leading cause of death in the world. More than 280,000 heart valves are replaced every year, usually because of stenosis, which is when the valve opening narrows and hardens due to the deposition of minerals on it.
Heart valve stenosis causes the heart to work harder to pump blood and increases the risk of experiencing heart failure, stroke or sudden cardiac death. Surgery is therefore the only possible solution.
Heart valves are replaced when mineral deposits impede their functioning. (Shutterstock)
We recently published an article in the journal Acta Biomaterialia where we presented our findings from analyzing 33 heart valves from patients that had to undergo surgery due to severe stenosis. Surprisingly, we found that the type of minerals present in the valves of men and women was different in composition and shape, and that the minerals were growing slower in women than in men.
If confirmed on a larger scale, the findings of these preliminary results could help researchers and doctors develop new techniques to diagnose and treat the diseases differently depending on the patient’s sex.
Diagnosing valve disease
Valve disease progression is usually monitored with scans that allow doctors to evaluate the the extent of mineral deposition, measured as the degree of “hardness” of the valve. Our finding that minerals develop slower in women than in men is in line with another previously known factor: for the same degree of severity of stenosis, women have less mineral deposits than men. This means that women’s diseases will be detected always at later stages than men, unless we come up with new techniques for diagnosis that take this difference into consideration.
The most striking difference we found was that one type of mineral was present almost exclusively in women’s valves. Minerals deposited in heart valves are a mixture of calcium and phosphate ions in various proportions. The most abundant mineral is called hydroxyapatite, and it is pretty much the same that we have in our bones and teeth.
Several men’s valves almost exclusively showed the presence of hydroxyapatite. But 85 per cent of the women’s valves we analyzed also included a mineral called dicalcium phosphate dihydrate, which is usually stable in more acidic environments than hydroxyapatite. For contrast, only 15 per cent of the men’s valves we analyzed showed traces of dicalcium phosphate dihydrate in their composition.
While we don’t know why this happened, the difference is striking and worth exploring further, especially because dicalcium phosphate dihydrate is more soluble than hydroxyapatite. This means that it may be easier to treat the calcifications found in women if targeted approaches are adopted.
Artificial heart valves frequently require replacement because the build up of mineral deposits can restrict their efficiency. (Shutterstock)
Synchrotron analysis
We were able to find these differences between women’s and men’s calcifications thanks to two factors: we tackled the mineral analysis with a technique that no other researchers in the field had used, and we were lucky to collaborate with Adel Schwertani, a cardiac surgeon in the Division of Experimental Medicine at McGill University, who gave us samples from both men and women to analyze.
The technique is called Near Edge X-Ray Fine Structure Spectroscopy (NEXAFS). It can only be done at a synchrotron, a large machine that spins electrons in circles several kilometres in diametre and, while they travel, generate light that can be used to analyze materials.
There are only a handful of synchotron research facilities around the world, and we went to the Canadian Light Source. We have been using the Saskatchewan-based research facility for several years to analyze different types of pathological calcifications.
NEXAFS is an excellent technique for examining pathological calcifications because these calcifications contain minerals that are quite similar to one another and are often not crystalline, which makes them hard to distinguish using other characterization techniques.
Surprising discoveries
The collaboration with Dr. Schwertani and doctoral student Kashif Khan has been crucial because they have provided us with samples that included a range of patients with different conditions, age and sex.
Had we analyzed a less diverse range of patients, we would not have found these results; we did not start from the hypothesis that there were sex-related differences. The findings were a surprise to us, and they were possible by the inclusion of diversity in project design.
This was a crucial takeaway for us, and is in line with research that shows that including diversity improves research quality. In health-related research, sample diversity can improve our understanding of disease mechanism and help develop new approaches to diagnostic and treatment of diseases.
In our future work, we would like to better understand the meaning and the mechanism behind these sex-related differences: using cell-culture and animal models, we will explore possible explanations for the difference and test different diagnostic and treatment methods.
Clearly, diversity of cell sources and animals will be the number one design aspect of this study.
![]()
Marta Cerruti, Associate Professor, Materials Engineering, McGill University and Ophélie Gourgas, Postdoctoral fellow, Medicine, McGill University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Voices of the RSC” is a series of written interventions from Members of the Royal Society of Canada. The articles provide timely looks at matters of importance to Canadians, expressed by the emerging generation of Canada’s academic leadership. Opinions presented are those of the author(s), and do not necessarily reflect the views of the Royal Society of Canada.